Overview
KATHERINE was a randomized, multicenter, open-label trial of 1486 patients with HER2+ EBC. Patients were required to have had neoadjuvant taxane and trastuzumab-based therapy with residual invasive tumor in the breast and/or axillary lymph nodes. Patients received radiotherapy and/or hormonal therapy concurrent with study treatment. Breast tumor samples were required to show HER2 overexpression. Patients were randomized (1:1) to receive KADCYLA or trastuzumab.
Study Design
KADCYLA was given intravenously at 3.6 mg/kg on Day 1 of a 21-day cycle. Trastuzumab was given intravenously at 6 mg/kg of Day 1 of a 21-day cycle. Patients were treated with KADCYLA or trastuzumab for a total of 14 cycles unless there was a recurrence of disease, withdrawal of consent, or unacceptable toxicity. At the time of the major efficacy outcome analysis, median treatment duration was 10 months for both KADCYLA- and trastuzumab-treated patients.
Endpoints
Primary Endpoint:
- Invasive disease-free survival (iDFS). iDFS was defined as the time from the date of randomization to first occurrence of ipsilateral invasive breast tumor recurrence, ipsilateral local or regional invasive breast cancer recurrence, distant recurrence, contralateral invasive breast cancer, or death from any cause.
Secondary Endpoints:
- iDFS including second primary non-breast cancer
- Disease free survival (DFS)
- Overall survival (OS)
Baseline Characteristics
Patient demographics and baseline tumor characteristics were generally balanced between treatment arms.
- The median age was ~49 years (range 23-80 years).
- 73% were White, 9% were Asian, 6% were American Indian or Alaska Native, 3% were Black or African American.
- 99.7% of patients were women.
- Tumor prognostic characteristics were similar across study arms.
- The majority of patients (77%) had received an anthracycline-containing neoadjuvant chemotherapy regimen.
- 20% of patients received another HER2-targeted agent in addition to trastuzumab as a component of neoadjuvant therapy; 94% of these patients received pertuzumab.
Efficacy Results
Primary Endpoint Results
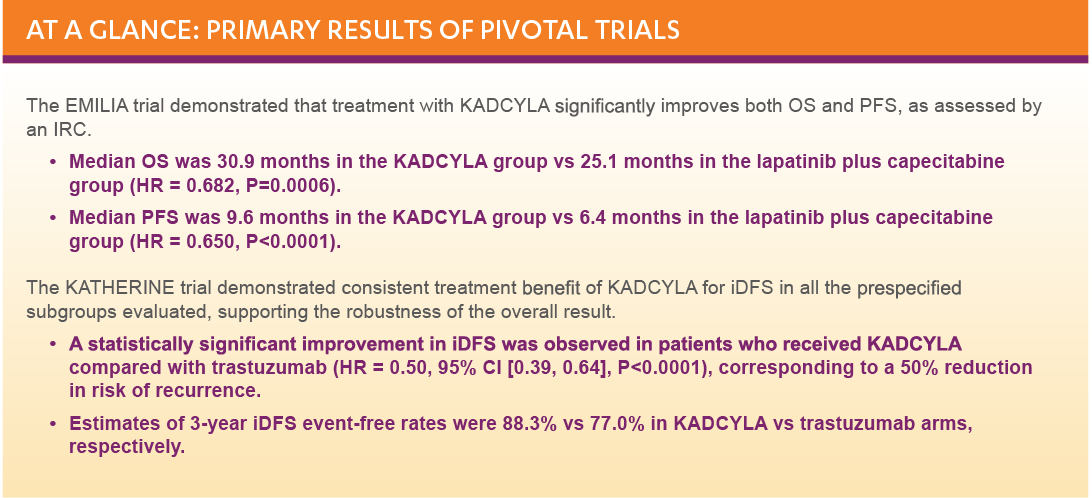
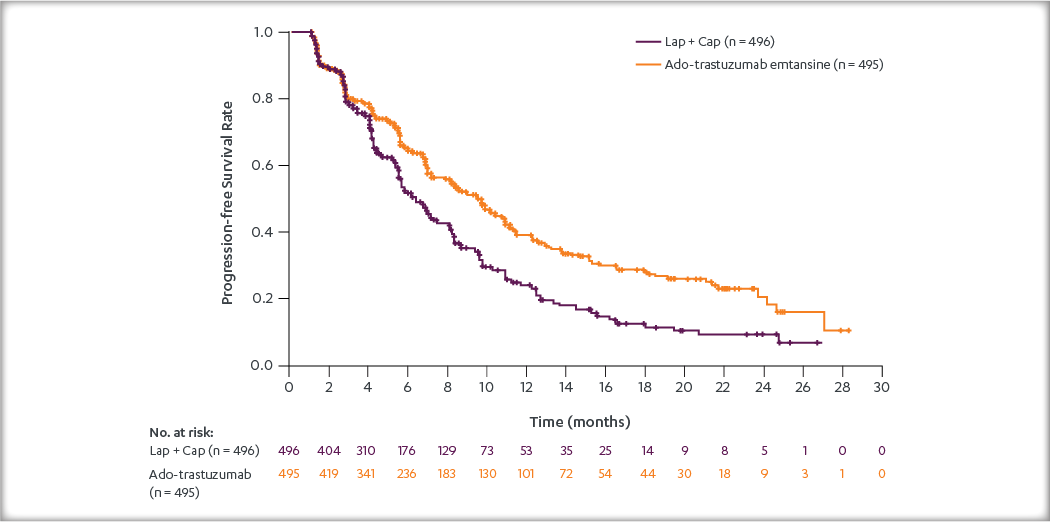
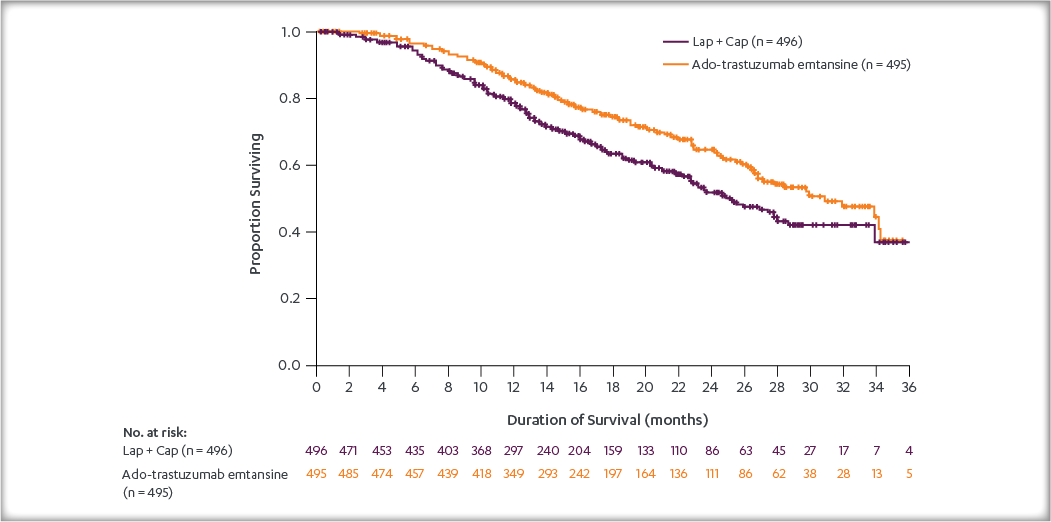
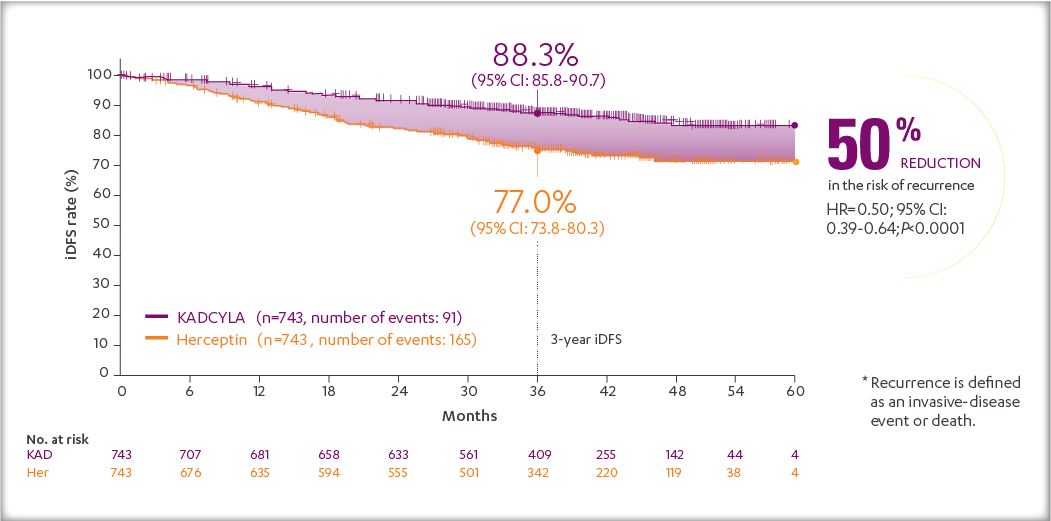
After a median follow-up of 40 months, there was a statistically significant improvement in iDFS in patients who received KADCYLA compared with trastuzumab. KADCYLA reduced the risk of recurrence by 50% versus trastuzumab (HR, 0.50 [95% CI: 0.39, 0.64; P < 0.0001)]. See the table and figure below for details.
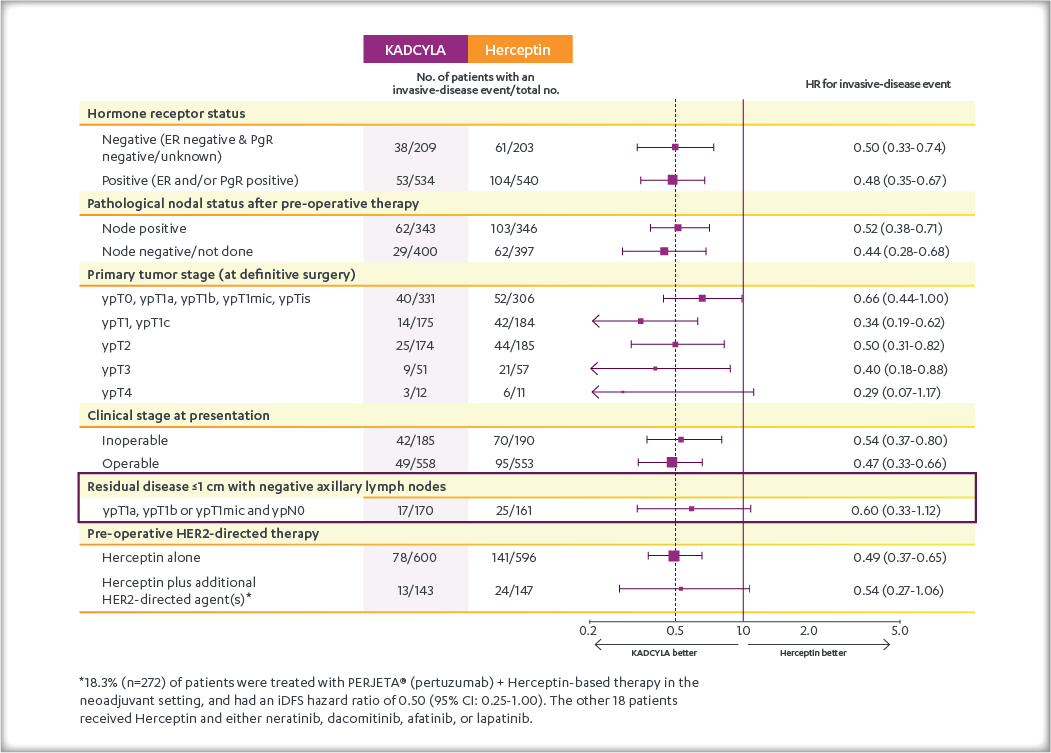
Consistent results were observed with KADCYLA in terms of iDFS across subgroups based on stratification factors, key baseline demographics and disease characteristics, and prior treatments.