The final sections of the Prescribing Information provide guidance on the storage and handling of KADCYLA (section 16) and review important counseling points (section 17).
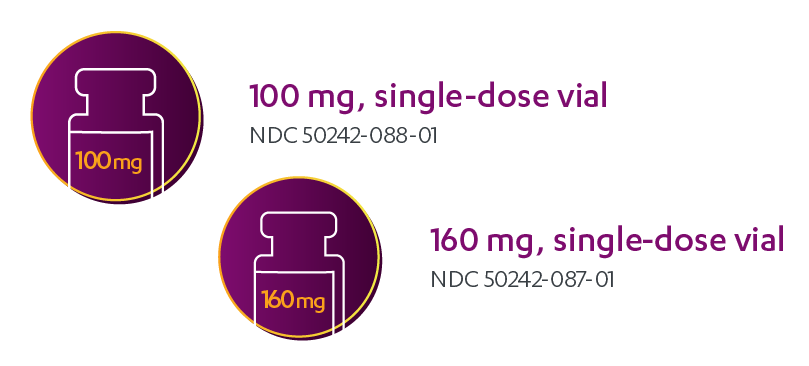
16.1 How Supplied/Storage and Handling
The packaging for KADCYLA is shown in
Figure 1. KADCYLA Packaging
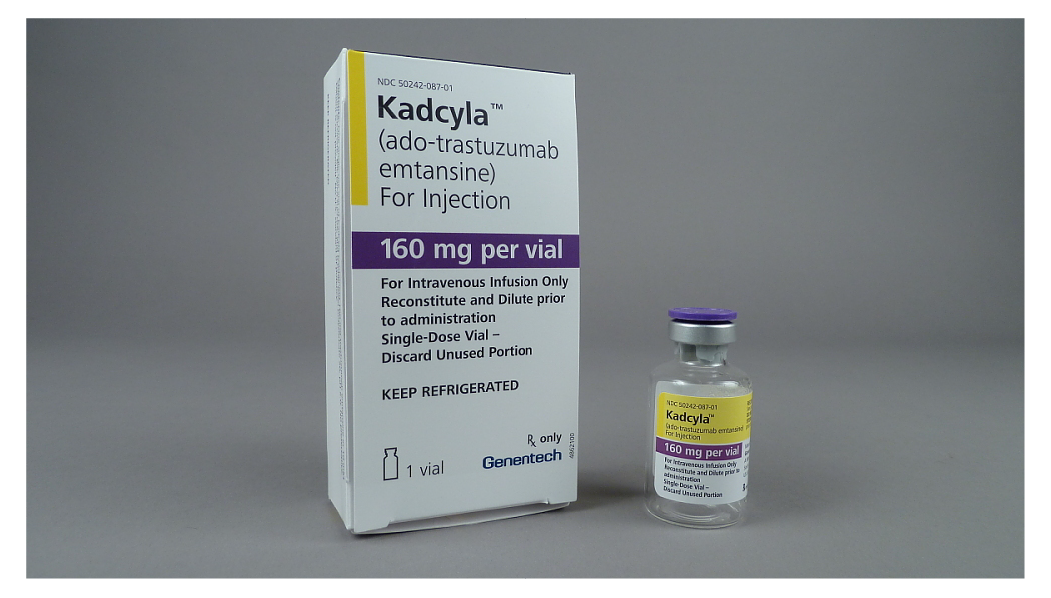
Figure 2. KADCYLA Vials and NDC Numbers