HIGHLIGHTS OF PRESCRIBING INFORMATION
The Highlights of Prescribing Information is a summary that can be found on the first page of all modern Package Inserts. It contains an overview of the most important contents of the PI, including any boxed warnings, a list of the most recent major changes to the PI, indications, dosing and administration, and safety information. Providers can use this page as a quick reference when needed.
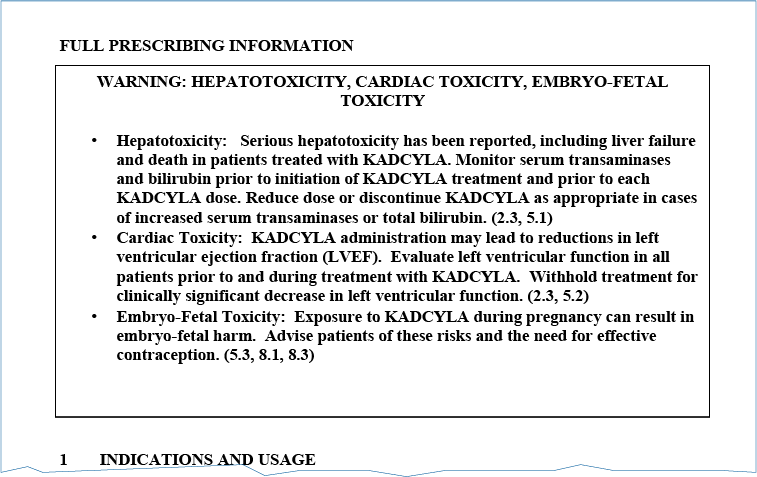
Boxed Warning
Boxed warnings, which are also referred to as black box warnings, alert prescribers to potential serious adverse reactions that may occur with a drug or restrictions necessary for the safe use of a drug. Providers should be aware that KADCYLA has a boxed warning for hepatotoxicity, cardiac toxicity, and embryo-fetal toxicity. It is important that you are aware of the boxed warning and are prepared to address any questions posed by your customers. More information on the adverse reactions mentioned in the boxed warning is provided in the Warnings and Precautions section.
Select the tabs below to see the Highlights of Prescribing Information summary page for KADCYLA and the boxed warning.