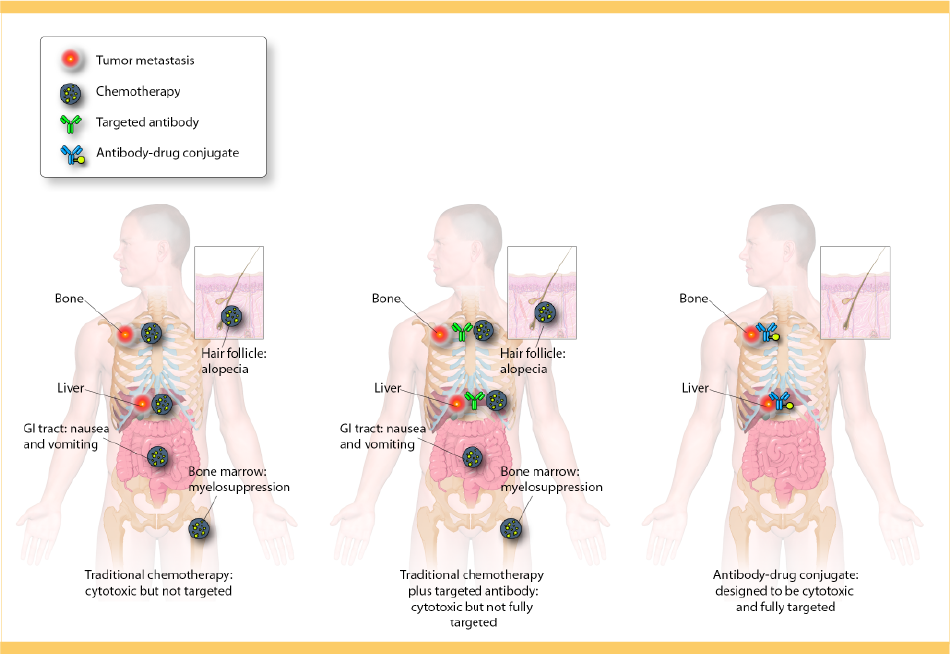
Approximately 15% to 25% of breast cancers have overexpression of the human epidermal growth factor receptor 2 (HER2) protein, increased HER2 gene amplification, or both. These breast cancers are called HER2-positive (HER2+) and are characterized by aggressive growth, greater likelihood to spread, and poor prognosis. There are, however, treatment options designed specifically for HER2+ breast cancer.
KADCYLA (ado-trastuzumab emtansine) is a HER2-targeted antibody-drug conjugate (ADC) that combines the antibody effects of HERCEPTIN (trastuzumab) with the cytotoxic effects of DM1. These two entities, which are linked together by a molecule called MCC, are designed to target HER2+ cells directly. Together, MCC and DM1 comprise emtansine. KADCYLA is the first ADC designed to target a solid tumor, and it represents a major advancement in oncology therapy.