1. Indications and Usage
The Indications and Usage section of the KADCYLA Prescribing Information is organized by clinical setting: Subsection 1.1 describes the indication for metastatic breast cancer(MBC), and Subsection 1.2 describes the indication for early breast cancer (EBC), which is defined as cancer that has not spread beyond the breast or axillary lymph nodes.
Metastatic Breast Cancer (MBC)
KADCYLA, as a single agent, is indicated for the treatment of patients with HER2+ metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either:
- Received prior therapy for metastatic disease, or
- Developed disease recurrence during or within 6 months of completing adjuvant therapy
Patients should be selected for therapy based on an FDA-approved companion diagnostic for KADCYLA.
Early Breast Cancer (EBC)
KADCYLA, as a single agent, is indicated for the adjuvant treatment of patients with HER2+ early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based therapy. Patients should be selected for therapy based on an FDA-approved companion diagnostic for KADCYLA.


Selected NCCN Guidelines: HER2+ EBC
PERJETA (pertuzumab) is indicated for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2+ MBC who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. Pertuzumab is also indicated for use in combination with trastuzumab and chemotherapy for the neoadjuvant treatment of patients with HER2+, locally advanced, inflammatory, or early stage breast cancer (either >2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer; and as adjuvant treatment for patients with HER2+ EBC at high risk of recurrence.
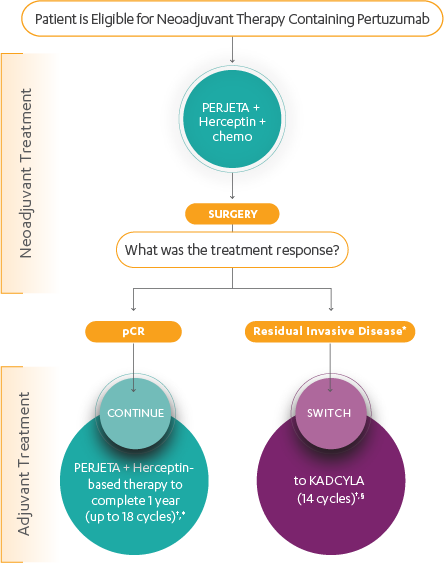
According to National Comprehensive Cancer Center (NCCN) guidelines, patients with HER2+ EBC should be treated with neoadjuvant systemic therapy incorporating trastuzumab. A pertuzumab-containing neoadjuvant regimen may be administered to patients with ≥T2 (ie, 2–5 cm) or node-positive, HER2+ EBC.
NCCN guidelines also recommend that patients who receive neoadjuvant therapy (with or without pertuzumab) then receive HER2-directed therapy in the adjuvant setting, with the choice of therapy directed according to their response to neoadjuvant treatment.
- No residual disease–patients with pathologic complete response (pCR) should continue adjuvant trastuzumab, with or without pertuzumab, to complete 1 year of HER2-directed therapy
- Residual disease–patients with residual disease should receive treatment with KADCYLA for 14 cycles. No further chemotherapy is administered with or after KADCYLA.

Selection of Adjuvant Systemic Therapy After Neoadjuvant Systemic Therapy